Part II.II Addendum: Pharmaceutical Biomanufacturing Scope & Scale
How do you manufacture biological drugs like mRNA vaccines and cell therapies? In big metal vats. The global capacity of such vats is estimated to be over 17 million liters across 1.6 thousand facilities, with two-thirds of that concentrated in America and Europe.
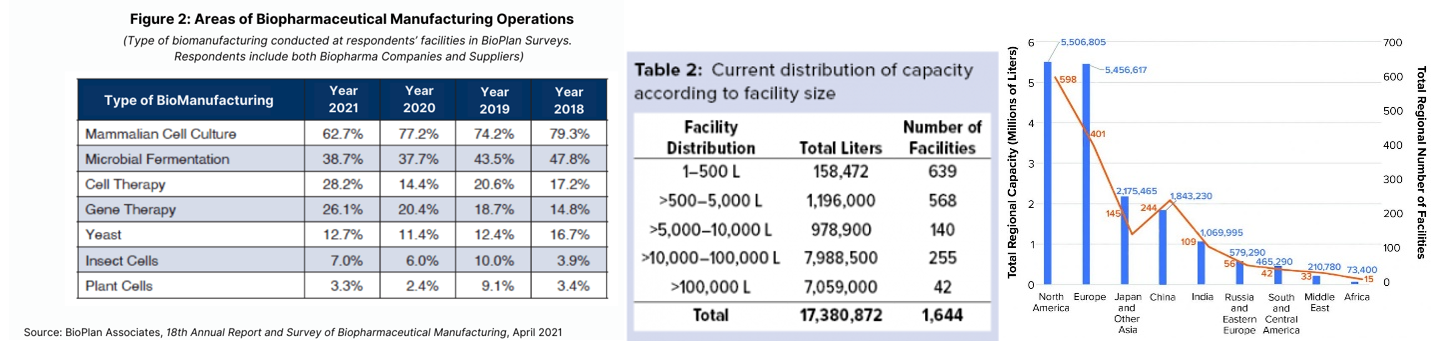
Here’s some key trends within the industry:
- Increasing focus on specialization and outsourcing with the proportion of companies outsourcing some part of their process rising from 42% in 2006 to 65% today
- Rising productivity with less frequent batch failures and shifts towards single-use batches from large stainless-steel tanks and perfusion systems from fed-back cultures. The former shortens processing times and time-to-market, enables modular manufacturing units, lowers risk of cross-contamination which allows manufacturing of multiple products at same facility, and reduced capex. Meanwhile, see the quote below for benefits of perfusion systems:
Perfusion culture has significant advantages over fed-batch culture regarding yield, quality, flexibility, and cost-effectiveness. A well-developed perfusion culture system—such as the WuXi Biologics Ultra-High Productivity Platform, or WuXiUP™—could enable 5–10-fold improvement on the cell density and productivity of almost any type of biologic, compared to a fed-batch culture. Additionally, utilizing continuous product harvest reduces the residence time of the product within the bioreactor, leading to improved product quality.
- See here for details on mRNA manufacturing specifically
Read next section: food & drink